Clinical and Economic Impact of Sickle Cell Disease
Gene therapies have the potential to revolutionize medicine and improve the lives of individuals living with sickle cell disease (SCD). Additional considerations such as high costs for these therapies and questions regarding insurance coverage remain major challenges to patient accessibility and further development.
The Clinical and Economic Impact Analysis (CEIA) Consortium
The Cure Sickle Cell Initiative (CureSCi) formed the Clinical and Economic Impact Analysis (CEIA) Consortium in 2019 to help identify and evaluate the cost effectiveness of gene therapies for SCD. The Consortium was funded by the National Heart, Lung, and Blood Institute (NHLBI) through awards to the University of Washington (UW) and the Fred Hutchinson Cancer Center (FH). The investigators from both institutions received feedback and guidance from an expert panel consisting of individuals living with sickle cell disease, advocates, clinicians with expertise in pediatric and adult sickle cell disease care and bone marrow transplant, as well as bioethicists, health economists, database analysts, and members of the payer community.
CEIA Mission
The CEIA’s mission was to develop simulation models that can be used to demonstrate the potential national impact of specific curative therapies for SCD, and the distribution of that impact on payers, employers, and families over the lifetime of the patients.
Models for Economic Analysis of SCD
Two independently developed simulation models were designed to assess lifetime outcomes with and without gene therapy for people living with SCD. The FH's Sickle Cell Disease Outcomes Research and Economics Model (FH-HISCORE) and the UW Model for Economic Analysis of Sickle Cell Cure (UW-MEASURE) were published in the Annals of Internal Medicine in January 2024.
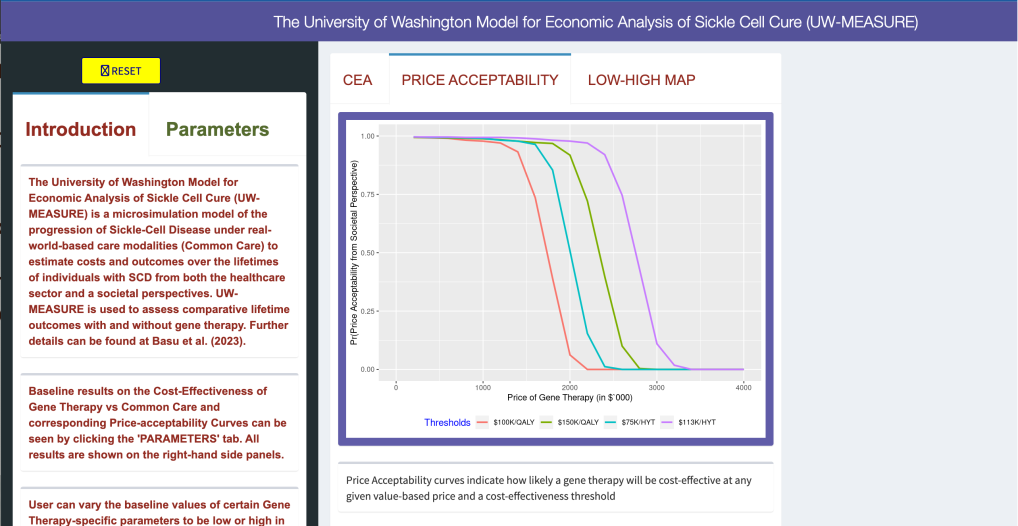
The UW-MEASURE microsimulation model web portal, which uses the R-Shiny platform, allows users to examine and adjust values that help to estimate healthcare and societal outcomes of SCD gene therapies.
“Both the UW and Fred Hutch models suggest that gene therapies can be life-altering for patients with sickle cell disease. We show prolonged, substantial reductions in pain crises events and reduced morbidity over time, greatly improving patients' prospects for long-term employment, decreasing or possibly eliminating caregiver burden, and substantially improving recipients' life expectancy and quality of life compared to current standards of care. The long-term durability of therapy is an important unknown that will impact cost-effectiveness. Future work comparing the clinical and economic effects of gene therapy versus stem cell transplantation will assist in guiding patients to the most appropriate and cost-effective therapy.”
SCOTT RAMSEY, MD, PHD
Principal Investigator
CEIA Goals
- Analyze the current SCD economic and standard-of-care landscape to inform the creation of two models that simulate real-world SCD care modalities and SCD disease progression.
- Use the models to perform clinical and cost-effectiveness analysis for payers/insurers that incorporate societal factors.
- Share findings through publicly available web-based simulation models, peer-reviewed publications, and conference presentations.
Anirban Basu, PhD
Health Economist at the Comparative Health Outcomes,Policy, and Economics (CHOICE) Institute, University of Washington, Seattle
Scott Ramsey, MD, PhD
Clinician and Health Economist at the Hutchinson Institute for Cancer Outcomes Research (HICOR), Fred Hutchinson Cancer Research Center
Beth Devine, PhD
Health Services Researcher at the Comparative Health Outcomes, Policy, and Economics (CHOICE) Institute, University of Washington, Seattle
Michael A. Bender, MD
Clinician and Sickle Cell Researcher at the Comparative Health Outcomes, Policy, and Economics (CHOICE) Institute, University of Washington, Seattle
Aaron Winn, PhD
Health Economist at the Hutchinson Institute for Cancer Outcomes Research (HICOR), Fred Hutchinson Cancer Research Center
Kate M. Johnson, PhD
The University of British Columbia (Formerly: Post-Doc, The CHOICE Institute, University of Washington, Seattle)
Boshen Jiao, PhD
University of Southern California (Formerly: PhD Student, The CHOICE Institute, University of Washington, Seattle)
SELECT ACTIVITIES
Performed a landscape analysis which included review of the following areas: population, interventions, comparators, outcomes, timing, setting (geographic), and study designs (PICOTS).
Conducted a literature search to:
- Better understand the burden of SCD (comorbidities, treatment complications, costs) and the cost-effectiveness of curative therapies compared to existing types of care.
- Inform parameters of economic models with most up-to-date values from available literature.
- Consider costs of curative therapies in other chronic disease areas.
Developed simulation models for SCD disease that:
- Incorporated epidemiology, biostatistics, decision analysis, and health policy.
- Used input parameters for costs, outcomes, and probabilities – to study how individuals with SCD progress over their lifetime.
- Utilized real-world evidence (registries, claims databases, electronic health records), or clinical trials.
- Validated against observed outcomes for individuals living with SCD. Can be used to project long-term health outcomes and costs.
Conducted an analysis of healthcare claims using Medicare, Medicaid, and Market Scan insurance claims records.
- Identified SCD patients with public and commercial health plan insurance coverage from 2008-2016.
- Analyzed data to identify the following by age group: SCD complication rates, healthcare resource use, and direct medical expenditures.
- Utilized information to help understand the SCD lifetime burden of illness overall and by key sub-groups (e.g. age, disease severity).
Developed a clinical and cost-effectiveness analysis using the University of Washington Model for Economic Analysis of Sickle Cell Cure (UW-MEASURE), and the Fred Hutchinson Institute Sickle Cell Disease Outcomes Research and Economics Model (FH-HISCORE). UW-MEASURE, which is used to assess comparative lifetime outcomes with and without gene therapy can be found here.
Featured Publication
As new gene therapies for sickle cell disease (SCD) are being considered by people living with the disease, one major consideration is cost. A recent analysis of the cost effectiveness of gene therapy for SCD found that it will likely be a cost-effective option compared to conventional treatments given during a patient’s lifetime – if the price stays under $2 million per person. The study, funded by the NHLBI’s Cure Sickle Cell Initiative, used two independent computer simulation models and provides the most detailed estimates to date of the current value of gene therapy treatments. The cost-effectiveness simulation models included the University of Washington Model for Economic Analysis of Sickle Cell Cure (UW-MEASURE) and the Fred Hutchinson Institute Sickle Cell Disease Outcomes Research and Economics Model (FH-HISCORE). Both models, which differ slightly in their design, analyzed insurance claims data on individuals with sickle cell disease who were enrolled in Medicaid, Medicare, or both. In addition to cost estimates, the models also focused on various health impacts, including quality of life and life expectancy, loss of time and productivity, and the impact on the patient’s caregiver and family.
- Jiao B, Basu A, Roth J, Bender M, Rovira I, Clemons T, Quach D, Ramsey S, Devine B. The Use of Cost-Effectiveness Analysis in Sickle Cell Disease: A Critical Review of the Literature. Pharmacoeconomics. 2021 Nov;39 (11): 1225-1241. doi: 10.1007/s40273-021-01072-z. Epub 2021 Aug 9. PMID: 34368937.
- Jiao B, Basu A, Ramsey S, Roth J, Bender MA, Quach D, Devine B. Health State Utilities for Sickle Cell Disease: A Catalog Prepared From a Systematic Review. Value Health. 2022 Feb;25(2):276-287. doi: 10.1016/j.jval.2021.08.002. Epub 2021 Sep 4. PMID:35094801; PMCID: PMC8804335.
- Baldwin Z, Jiao B, Basu A, Roth J, Bender MA, Elsisi Z, Johnson KM, Cousin E, Ramsey SD, Devine B. Medical and Non-medical Costs of Sickle Cell Disease and Treatments from a US Perspective: A Systematic Review and Landscape Analysis. Pharmacoecon Open. 2022 Jul;6(4):469-481. doi: 10.1007/s41669-022-00330-w. Epub 2022 Apr 26.PMID: 35471578; PMCID: PMC9283624.
- Quach D, Jiao B, Basu A, Bender MA, Hankins J, Ramsey S, Devine B. A landscape analysis and discussion of value of gene therapies for sickle cell disease. Expert Rev Pharmacoecon Outcomes Res. 2022 Sep;22(6):891-911. doi: 10.1080/14737167.2022.2060823. Epub 2022 Apr 18. PMID: 35363602.
- Johnson KM, Jiao B, Ramsey SD, Bender MA, Devine B, Basu A. Lifetime medical costs attributable to sickle cell disease among nonelderly individuals with commercial insurance. Blood Adv. 2023 Feb 14;7(3):365-374. doi:10.1182/bloodadvances.2021006281. PMID: 35575558; PMCID: PMC9898623.
- Johnson KM, Jiao B, Bender MA, Ramsey SD, Devine B, Basu A. Development of a conceptual model for evaluating new non-curative and curative therapies for sickle cell disease. PLoS One. 2022 Apr 28;17(4):e0267448. doi: 10.1371/journal.pone.0267448. PMID: 35482721; PMCID: PMC9049306.
- Jiao B, Hankins JS, Devine B, Barton M, Bender M, Basu A. Application of validated mapping algorithms between generic PedsQL scores and utility values to individuals with sickle cell disease. Qual Life Res. 2022 Sep;31(9):2729-2738. doi: 10.1007/s11136-022-03167-2. Epub 2022 Jun 17. PMID: 35715626.
- Ramsey SD, Bender MA, Li L, Johnson KM, Jiao B, Devine B, Basu A. Prevalence of comorbidities associated with sickle cell disease among non-elderly individuals with commercial insurance - A retrospective cohort study. PLoS One. 2022 Nov 29;17(11):e0278137. doi: 10.1371/journal.pone.0278137. PMID: 36445914; PMCID: PMC9707783.
- Winn A, Basu A, Ramsey SD. A Framework for a Health Economic Evaluation Model for Patients with Sickle Cell Disease to Estimate the Value of New Treatments in the United States of America. Pharmacoecon Open. 2023 Mar;7(2):313-320. doi: 10.1007/s41669-023-00390-6. Epub 2023 Feb 11. PMID: 36773220; PMCID: PMC10043085.
- Jiao B, Basu A. Associating Health-Related Quality-of-Life Score with Time Uses to Inform Productivity Measures in Cost-Effectiveness Analysis. Pharmacoeconomics. 2023 Mar 6. doi: 10.1007/s40273-023-01246-x. Epub ahead of print. PMID: 36877451.
- Boshen Jiao, Kate M. Johnson, Scott D. Ramsey, M. A. Bender, Beth Devine, Anirban Basu. Long-Term Survival of Individuals with Sickle Cell Disease: A Nationwide
Cohort Study of Medicare and Medicaid Beneficiaries. Blood Adv. 2023 Mar 16;bloodadvances.2022009202. doi: 10.1182/bloodadvances.2022009202. PMID: 3692916



 The Clinical and Economic Impact Analysis (CEIA) Consortium: A Conversation with Dr. Anirban Basu
The Clinical and Economic Impact Analysis (CEIA) Consortium: A Conversation with Dr. Anirban Basu