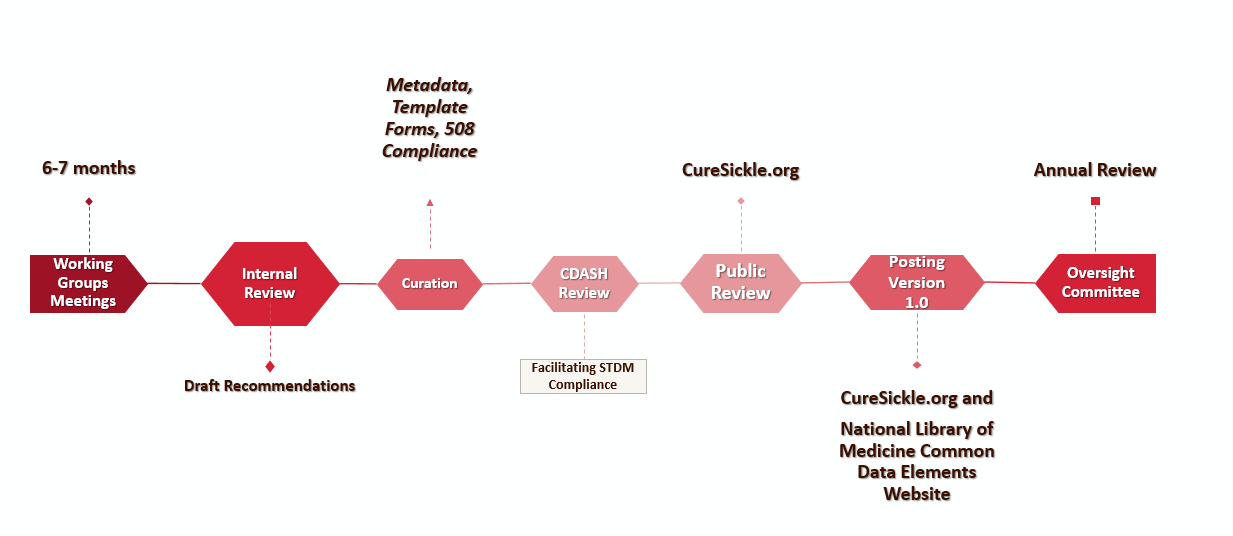
Working groups were formed to review five clinical research study domains:
- Genetics/Assays;
- Physical Examination/Medical History;
- Cardiopulmonary, Renal and Cerebrovascular;
- Outcomes; and
- Monitoring Side Effects.
These working groups were comprised of SCD adult and pediatric specialists, patient advocates, genetic therapy specialists, neuropsychologists, nurses, data managers, manufacturers, industry members, and academics. After a Public Review period and follow-up with working groups, version 1.0 of the standardized data forms have been released. The forms will also be made available through the National Library of Medicine (NLM) website. This will be an iterative process, and feedback from users will be encouraged.
Cardiopulmonary, Renal and Cerebrovascular Working Group
Michael DeBaun, MD, MPH (Co-Chair)
Vanderbilt University Medical Center, Nashville, Tennessee
Alexis Thompson, MD, MPH (Co-Chair)
Northwestern University, Chicago, Illinois
Anjulika Chawla, MD
bluebird bio, Cambridge, Massachusetts
Robyn Cohen, MD
Boston Medical Center, Boston, Massachusetts
Courtney Fitzhugh, MD
National Heart, Lung and Blood Institute, Bethesda, Maryland
Lisa Garrett, RN, CCRP
Washington University in St. Louis, St. Louis, Missouri
Eileen Hansbury, PA
Boston Children’s Hospital, Boston, Massachusetts
Tabitha Hendershot
Research Triangle Institute, Washington, DC
Kathleen Hewitt, DND, RN, CPNQ
American Society of Hematology Research Collaborative, Gainesville, Virginia
Lori Jordan, MD, PhD
Vanderbilt Unversity Medical Center, Nashville, Tennessee
Gregory Kato, MD
CSL Behring, King of Prussia, Pennsylvania
Chava Kimchi-Sartay, PhD
Food and Drug Administration, Silver Spring, Maryland
Allison King, MD, MPH, PhD
Washington University of St. Louis, St. Louis, Missouri
Elizabeth S. Klings, MD
Boston University School of Medicine, Boston, Massachusetts
Lakshmanan Krishnamurti, MD
Emory University School of Medicine, Atlanta, Georgia
Jeffrey Lebensburger, DO
University of Alabama Birmingham, Birmingham, Alabama
Robert Lindblad, MD
Emmes, Rockville, Maryland
Charity Oyedeji, MD
Patient Advocate
Duke University, Durham, North Carolina
Vandana Sachdev, MD
National Heart, Lung and Blood Institute, Bethesda, Maryland
Santosh Saraf, MD
University of Illinois at Chicago, Chicago, Illinois
Genetics and Assays Working Group
Julie Kanter, MD (Co-Chair)
Medical University of South Carolina, Charleston, South Carolina
John Pierciey, MSc. (Co-Chair)
bluebird bio, Cambridge, Massachusetts
Gang Bao, PhD
Rice University, Houston, Texas
Francine Baker
Patient Advocate
Carlo Brugnara, MD
Boston Children’s Hospital, Boston, Massachusetts
Ashraf El Fiky, MBBCh, PhD
Emmes, Rockville, Maryland
Bindu George, MD
Food and Drug Administration, Silver Spring, Maryland
Joseph Gold, PhD
City of Hope, Duarte, California
Allison Intondi, PhD
Integral Medicines, San Francisco, California
Alison Kujawski, CHES
American Society of Gene & Cell Therapy, Washington, DC
LaTasha Lee, PhD, MPH
National Minority Quailty Forum, Washington, DC
John Manis, MD
Boston Children’s Hospital, Boston, Massachusetts
David C. Shyr, MD
Stanford Medicine, Palo Alto, California
John Tisdale, MD
National Institute of Health, Bethesda, Maryland
Monitoring Side Effects Working Group
Punam Malik, MD (Co-Chair)
Cincinnati Children's Hospital, Cincinnati, Ohio
Mark Walters, MD (Co-Chair)
University of California San Francisco, Oakland, California
Smita Bhatia, MD
University of Alabama at Birmingham, Birmingham, Alabama
Charles Chesson, PhD
Amerian Society of Hematology Research Collaborative, Washington, DC
Nancy Di Fronzo, PhD
National Institute of Health, Bethesda, Maryland
Mary Eapen, MRCPI, MS, MBBS
Medical College of Wisconsin, Milwaukee, Wisconsin
Barbara Kroner, PhD
Research Triangle Institute, Durham, North Carolina
Navneet Majhail, MD, MS
Cleveland Clinic, Cleveland, Ohio
Joseph McIntosh, MD
Aruvant Sciences, Inc., Basel, Switzerland
Jeremy Pantin, MD
HCA Healthcare, Nashville, Tennessee
Lydia Harley Pecker, MD
Johns Hopkins University, Baltimore, Maryland
Katy Rezvani, MD, PhD
The University of Texas, Houston, Texas
Shalini Shenoy, MD
Washington University School of Medicine, St. Louis, Missouri
Patrick Carroll, MD (Chair)
Johns Hopkins Medicine, Baltimore, Maryland
Francine Baker
Patient Advocate
M. A. Bender, MD, PhD
University of Washington, Seattle, Washington
Andres Brainsky, MD
CSL Behring, King of Prussia, Pennsylvania
Amanda Brandow, DO
Medical College of Wisconsin, Milwaukee, Wisconsin
Traci Clemons, PhD
Emmes, Rockville, Maryland
Beth Devine, PhD, PharmD, MBA
University of Washington, Seattle, Washington
Meghan Gallagher
bluebird bio, Cambridge, Massachusetts
Susan Geyer, PhD
Mayo Clinic, Tampa, Florida
Gregory Kato, MD
CSL Behring, King of Prussia, Pennsylvania
Megha Kaushal, MD
Food and Drug Administration, Silver Spring, Maryland
Julie Panepinto, MD, MSPH, FAAP
Medical College of Wisconsin, Milwaukee, Wisconsin
Jennifer Popovic, DVM
Research Triangle Institute, Waltham, Massachusetts
Amy Sobota, MD, MPH
Boston University, Boston, Massachusetts
Marsha Treadwell, PhD
University of California San Francisco, Oakland, California
Shauna Whisenton
American Society of Hematology Research Collaborative, Silver Spring, Maryland
Teonna Woolford
Patient/Advocate
Physical Examination/Medical History Working Group
Sophie Lanzkron, MD, MHS (Co-Chair)
Johns Hopkins School of Medicine, Balitmore, Maryland
Deepa Manwani, MD (Co-Chair)
Albert Einstein College of Medicine, Bronx, New York
Anirban Basu, PhD
University of Washington, Seattle, Washington
Velvet Brown-Watts
Patient Advocate/Caregiver
Victoria Coleman-Cowger, PhD
Emmes, Rockville, Maryland
Nahed El Kassar, MD, PhD
National Heart, Lung and Blood Institute, Bethesda, Maryland
Lisa Garrett, RN, CCRP
Washington University in St. Louis, St. Louis, Missouri
Stephanie Guarino, MD
Nemours/Alfred I. duPont Hospital for Children, Wilmington, Delaware
Kavita Natrajan, MD
Food and Drug Administration , Silver Spring, Maryland
Helen Pan, PhD
Research Triangle Institute, Research Triangle Park, North Carolina
Robert Plovnick, MD, MS
American Society of Hematology, Washington, DC
Patricia Steinert, PhD, MBA
Center for International Blood & Marrow Transplant Research, CIBMTR®, Milwaukee, Wisconsin
Yvette Tanhehco, MD, PhD, MS
Columbia University Medical Center, New York, New York
Sara Vesely, PhD
The University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
Kesha Weeks
Patient Advocate/Caregiver
Traci Mondoro, PhD
National Heart, Lung and Blood Institute, Bethesda, Maryland
Elizabeth Wagner, MPH
National Heart, Lung and Blood Institute, Bethesda, Maryland
Lis Welniak, PhD
National Heart, Lung and Blood Institute, Bethesda, Maryland
Sherita Alai, MS
Emmes, Rockville, Maryland
Karen Hewitt
Emmes, Rockville, Maryland
Rebecca Johnson, PhD
Emmes, Rockville, Maryland
Dan Sinnett, PhD
Emmes, Rockville, Maryland
Glenn Tucker
Emmes, Rockville, Maryland